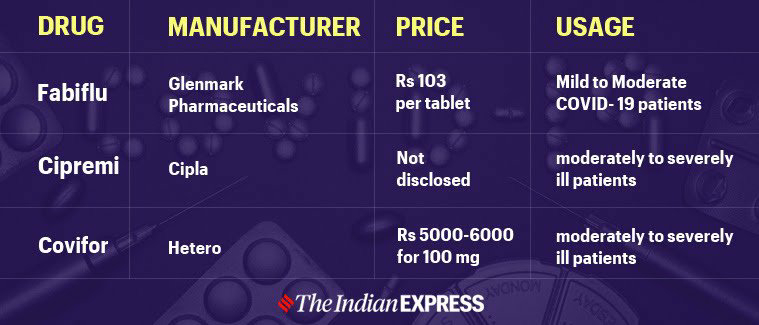
Drugs and Cosmetics Act 1940 and Rules 1945
The Drugs and Cosmetics Act of 1940 is an Indian law that governs the import, production, and distribution of drugs in the country. The act's principal goal is to ensure that pharmaceuticals and cosmetics sold in India are safe, effective, and meet state quality standards. The accompanying Drugs and Cosmetics Rules, 1945, contain requirements for classifying drugs into schedules, as well as requirements for storing, selling, displaying, and prescribing each category. History - chain of events British rule – No proper healthcare infrastructure, no pharmacy rules Drug Enquiry Committee led by Chopra committee made observations and gave report and recommended Government of India to develop rules for conduct of Pharmacy Industry Drug and Cosmetics Act 1940 was introduced Drug and Cosmetics Rules under the Act, 1945 was introduced Rules and acts were extended to whole India Drugs and Cosmetics Act 1940 Drugs and Cosmetics Act, 1940 was introduced by Government of India to regulate the i...